The Importance of External Quality Assurance (EQA) in Medical Laboratories
Writer and Editor : Tomi Saputra Jaya
Case Study: Participation of Biometrik Medical Laboratory in EQA on June 20, 2025
In the world of medical laboratory services, the accuracy and reliability of test results are critical aspects. To ensure the quality of test results, laboratories rely not only on internal quality control but also need to participate in a broader and more objective quality evaluation system: the External Quality Assurance (EQA) program.
What is External Quality Assurance (EQA)?
EQA is a quality assessment program conducted by an independent external institution. It aims to evaluate laboratory performance through proficiency testing. In this program, laboratories receive test samples from a third party, analyze them according to standard procedures, and submit the results for evaluation.
Why is EQA Important for Laboratories?
1. Ensuring Test Result Accuracy
EQA helps laboratories evaluate whether their results meet national and international standards. This ensures that results provided to doctors or patients accurately reflect the patient’s condition.
2. Indicator of Laboratory Competence
Successful participation in EQA indicates a laboratory’s ability to work consistently and accurately. This builds trust among the public, medical institutions, and accreditation bodies.
3. Identifying and Correcting Errors
EQA results reveal procedural, technical, or systemic errors, allowing laboratories to implement corrective and preventive actions.
4. Accreditation Requirement
Standards like ISO 15189:2022 mandate EQA participation as part of a laboratory’s quality management system. Regular participation demonstrates commitment to quality and continuous improvement.
5. Improving Staff Competence
EQA encourages laboratory staff to adhere to correct procedures and stay updated with advancements in medical laboratory science and technology.
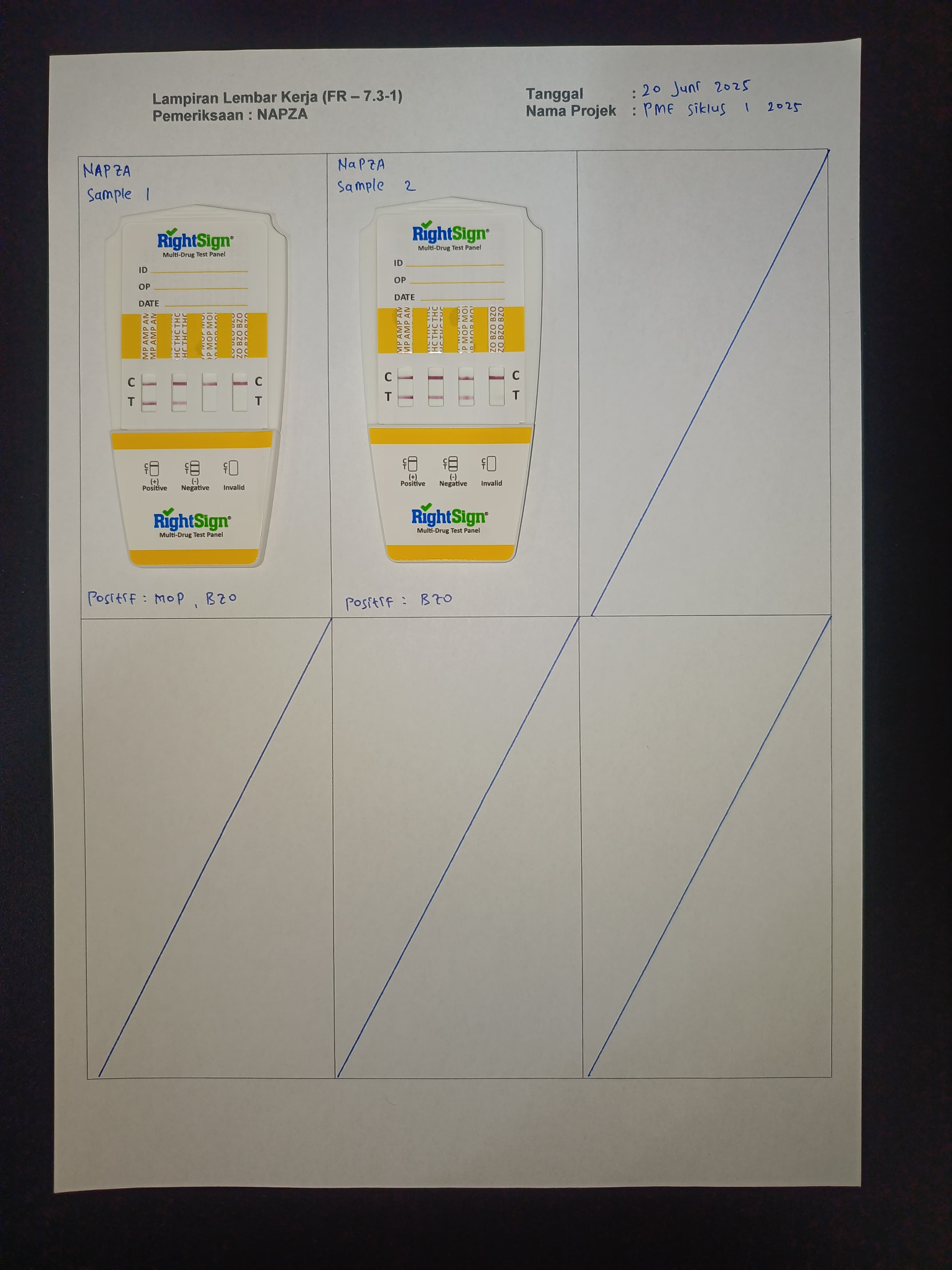
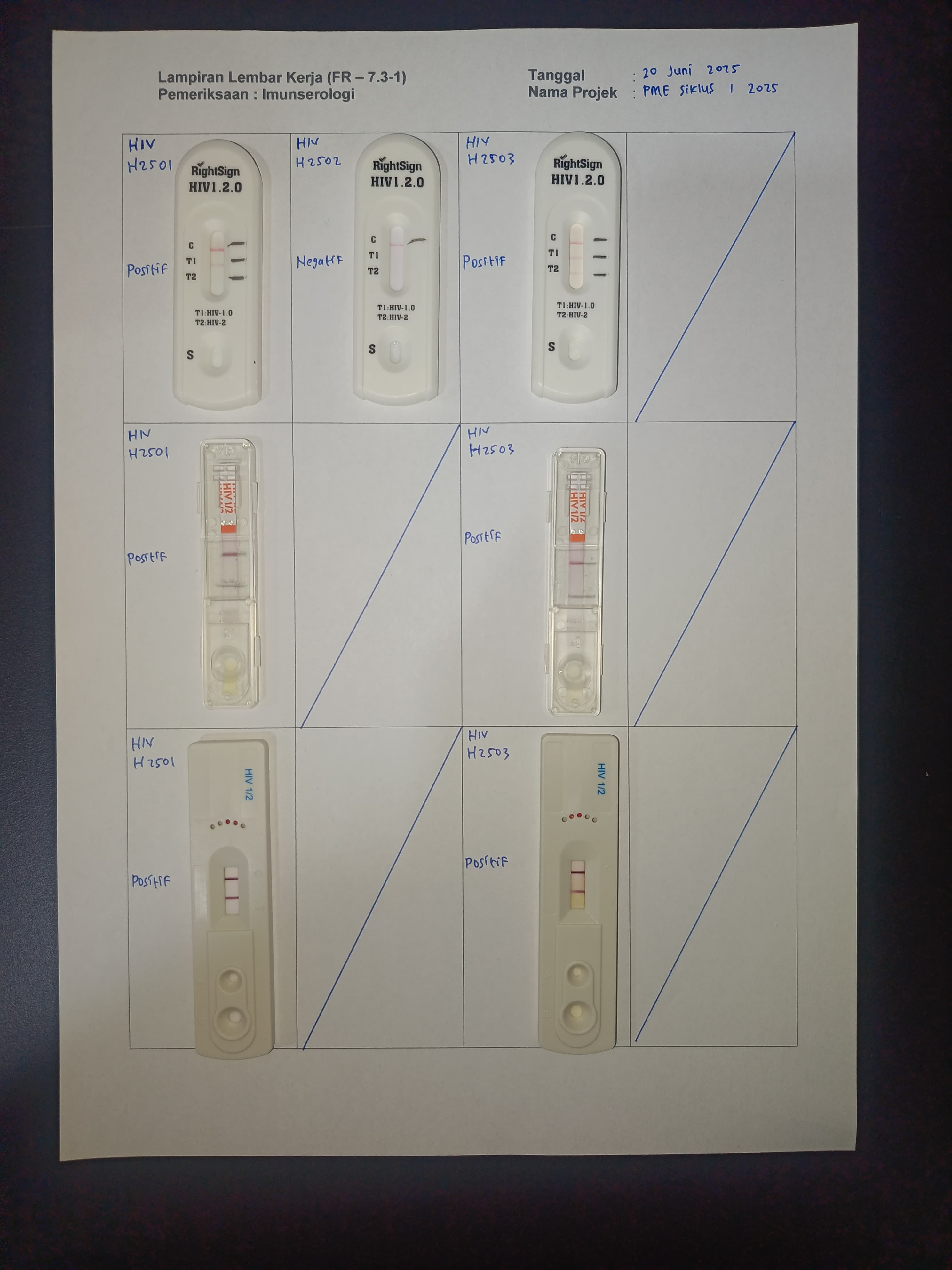
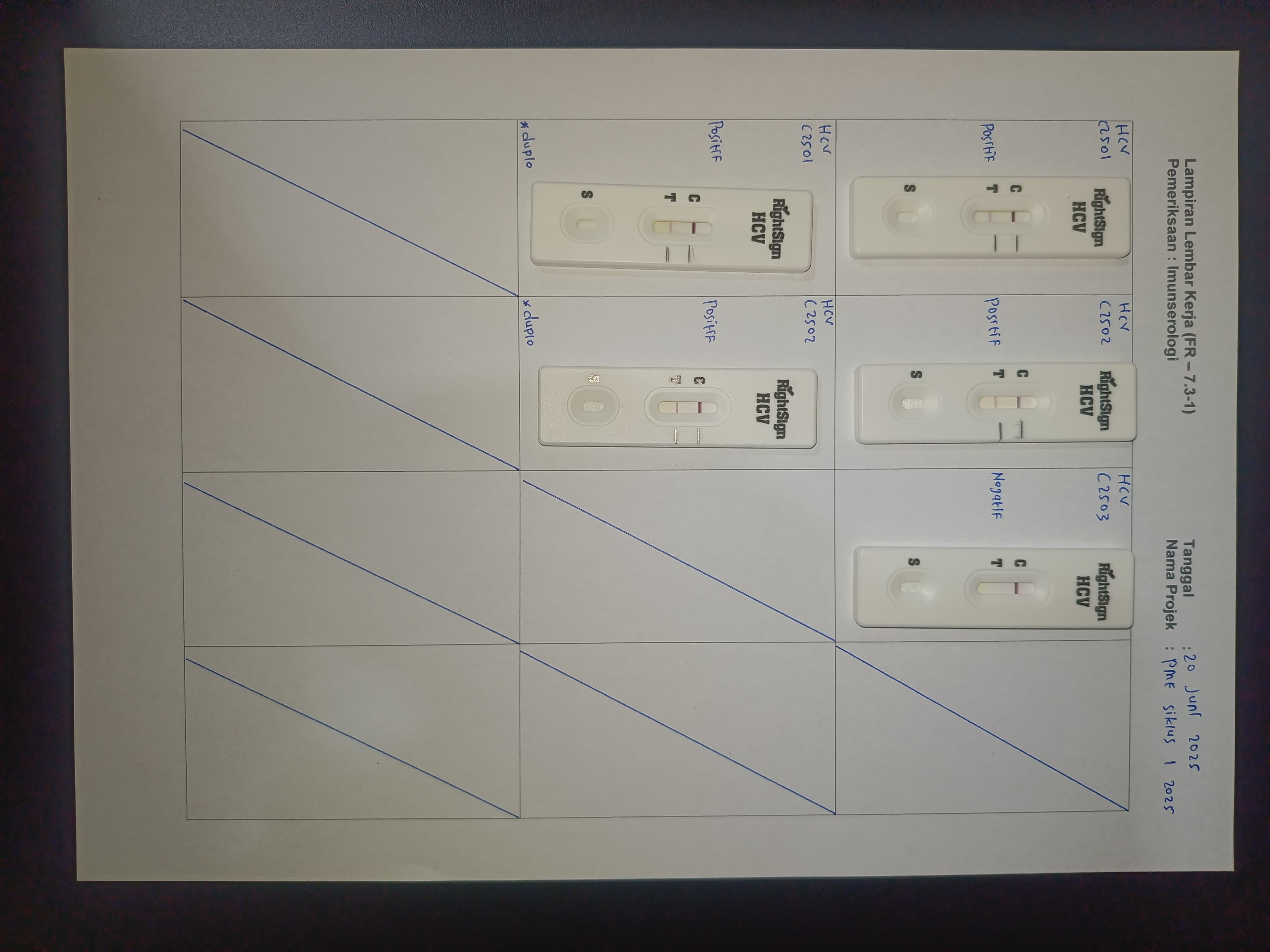
Participation of Biometrik Medical Laboratory in EQA on June 20, 2025
Upholding principles of quality and professionalism, Biometrik Laboratory participated in the External Quality Assurance program held on June 20, 2025. This reflects the laboratory’s commitment to ensuring reliable test results for patients and clinicians.
In this EQA cycle, Biometrik Laboratory received test panels from the organizer, performed tests according to applicable Standard Operating Procedures (SOPs), and submitted results for evaluation. The entire process was strictly supervised to ensure data accuracy and integrity.
Conclusion
External Quality Assurance is not merely an administrative obligation but a vital tool for maintaining and enhancing laboratory service quality. Through regular EQA participation—as demonstrated by Biometrik Laboratory on June 20, 2025—laboratories prove their dedication to quality, patient safety, and professionalism in medical laboratory practice.
Leave a comment