Improvement System for Bioequivalence Study
Writer by Intan Kumala Santriani, Editor by Oktaviani Utami Dewi
Depok, March 2nd 2023, biometrikriset.com - Along with the development of science and technology, software for processing bioequivalence parameter data has also progressed to support bioequivalence test centers in analyzing bioanalytical data. Currently, PT Biometrik Riset Indonesia has used Phoenix WinNonlin® Version 8.3 (Certara L.P., St. Louis, MO, USA) software as a statistical computer program that assists in processing bioanalytical data, instead of using EquivTest™ software previously. The use of WinNonlin® software makes Biometrik more confident to reach the international world, starting with ASEAN Harmonization.

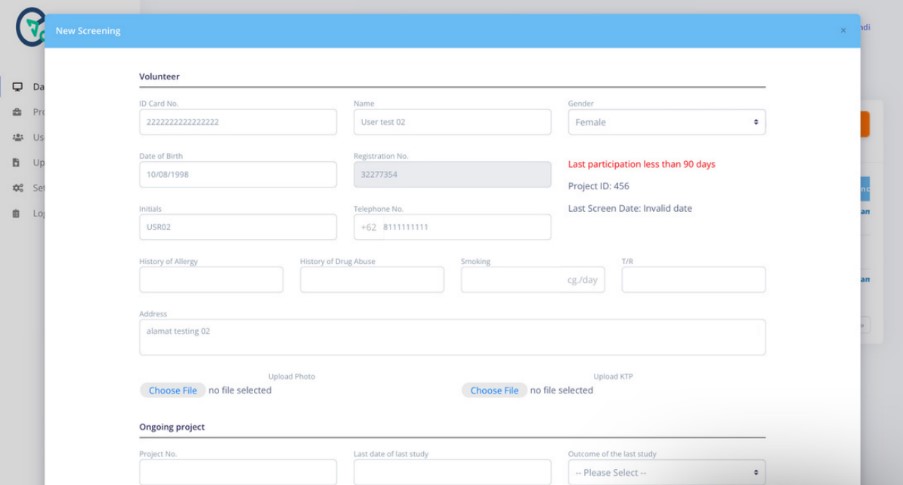
Besides that to fulfill the validity of Clinical Trials and Compliance with the Personal Data Protection Act (UU PDP No. 27/2022) and meet the requirement on guideline Bioequivalence study from BPOM (PerBPOM No. 11, 2022) for subject centralized database between Sentra Bioequivalence Study which are kept confidential. PT Biometrik Riset Indonesia conducted manual subject data migration by applying the software Ceksubjek.com as the digital volunteer recording system during the screening process of test subjects and to ensure that volunteers who participated in the bioequivalence study are more than 3 months.
"This is the important milestone Biometrik has used WinNonlin® for statistical calculations that are more reliable and accepted by all countries internationally. With the improvement system for bioequivalence study on Biometrik, we hope to increase the integrity of bioequivalence result and trust of sponsors to conduct bioequivalence study,”said Mrs. Effi as Director of PT Biometrik Riset Indonesia.
Leave a comment